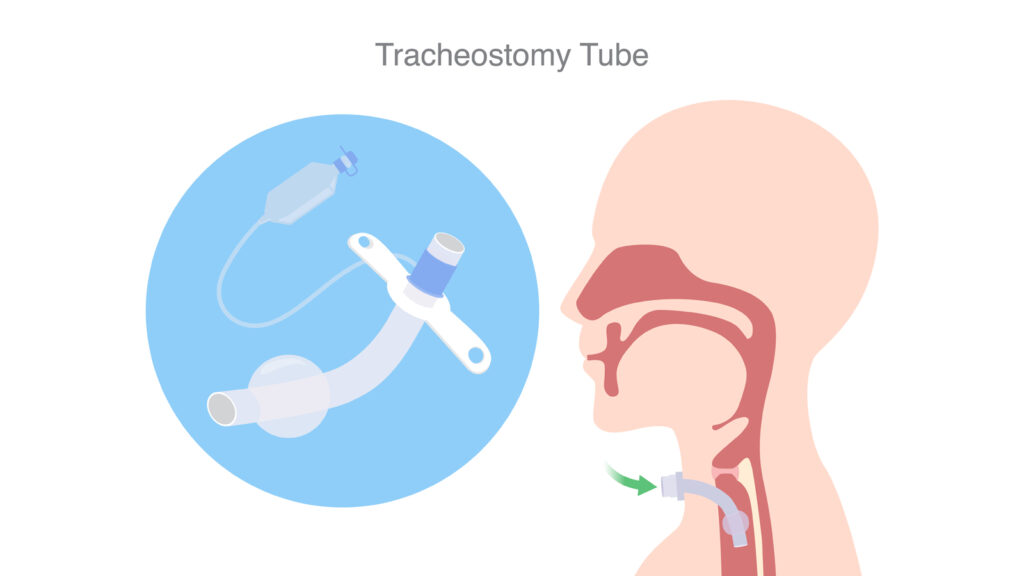
The U.S. Food and Drug Administration (FDA) has alerted patients, caregivers, and healthcare providers that there is a shortage of tracheostomy tubes, including Bivona tracheostomy tubes manufactured by ICU Medical. A shortage of Bivona tracheostomy tubes is more likely to impact pediatric patients because the supply of alternative tubes with similar functionality may be limited. The FDA is working closely with manufacturers and other stakeholders to help quickly resolve supply challenges and support availability of these critical devices for patients who need them. People who use these tubes, including caregivers and healthcare providers, should consider the FDA’s recommendations.